Transcranial Magnetic Stimulation for Persistent Depression
Safe, Effective & Medication-Free Treatment
- TMS therapy is covered by most insurance.
- TMS therapy is safe and FDA-cleared.
- Treatment typically takes 6 weeks.
“For about 20 minutes a day, five days a week, magnetic pulses targeted the part of my ‘sleeping’ brain that was making me sick. By the fourth week of treatment—a miracle! The storm cloud of depression started to lighten and lift. By the final week, it was gone completely. No depression; no anxiety! I was back! Happy, calm, strong. Excited about my life and the future.”
Facts About TMS
TMS is an advanced therapy that gently stimulates focused areas of the brain to eliminate persistent depression.
- TMS is a medication-free treatment; you won’t experience the usual unpleasant effects of anti-depressants.
- TMS is proven safe.
- The effectiveness of TMS has been studied for decades and has been FDA-cleared since 2008.
- TMS has no impact on memory or cognitive function.
- TMS is not ECT (electroconvulsive therapy).
What to Expect from TMS Treatment
Here’s a brief look at what you may experience at a TMS treatment session:
- You remain awake during treatment, seated in a comfortable chair while a magnet gently stimulates your brain.
- TMS treatments are painless and sessions lasting 15 minutes, with treatments being 3 minutes.
- TMS works by stimulating neurons in a focused area of the brain that impact moods and by strengthening connections to the brain where the impact can be permanent.
- TMS generally produces transformative results after about six weeks of treatment (20-minute sessions, five days a week).
"Thank you to the team at Anew Era TMS especially Kaitlyn, my therapist . I feel that this round of treatment has been life changing. I would recommend it to anyone, who wants a boost in their quality of life."
"Meeting with Kaitlyn every day was its own kind of therapy. She was always so kind and compassionate as I talked and talked. The TMS for anxiety changed my life in a way I didn't think was possible. I am off anti anxiety meds that I was on for 15 years"
When to Know It's Time for TMS
How do you know when it’s time to receive TMS treatment? Here’s what to consider:
- There is no change after trying multiple prescribed antidepressants.
- There is no change after using a combination of prescribed antidepressants and psychotherapy.
- An individual cannot tolerate antidepressant medications due to side-effects.
Approximately 5.5 million people who struggle with depression have reported that antidepressants didn’t give them relief from their symptoms.1,2,3 If you haven’t found relief from other persistent depression treatments, turn to the leading provider of TMS. DiscoveryMD is here to help.
“Overall, [TMS] has changed my life entirely… It’s like a brain fog has been lifted… The changes have been positive and constructive [after TMS treatment].”
Convenient Locations
Our goal is to make it easy and convenient for you to receive high-quality, science-backed treatment. TMS is offered at every DiscoveryMD location. Check our individual locations for the office that is closest to you and for details about all the services available.
FAQs about TMS
A magnetic field is created around the upper left part of your head (prefrontal cortex) to help stimulate neurons which in return helps with dopamine, serotonin and increases blood flow.
Those with persistent depression and major depressive disorder (MDD) who have not felt improvement with one or more medications are eligible for TMS treatment. There are other things TMS could help people overcome, though studies still need to be done. Several of these off-label items are awaiting approval and may not be covered by insurance. Some people may find relief from TMS for:
- Anxiety
- Obsessive compulsive disorder (OCD)
- Smoking
- Post-traumatic stress disorder (PTSD)
- Drug or alcohol cravings
- Parkinson’s disease
- Dystonia
- Tinnitus
- Spasticity
- Epilepsy
- Post-stroke pain/neuropathic pain
- Migraines
Yes, most major insurance companies cover this treatment, after getting prior authorization.
Not at all. TMS treatment is non-evasive, done daily between 10-30 minutes, there is no sedation is required, and you can take yourself to and from the appointments. You can come during your lunch break at work, then go on your way! There are no side effects like the ones experienced from ECT.
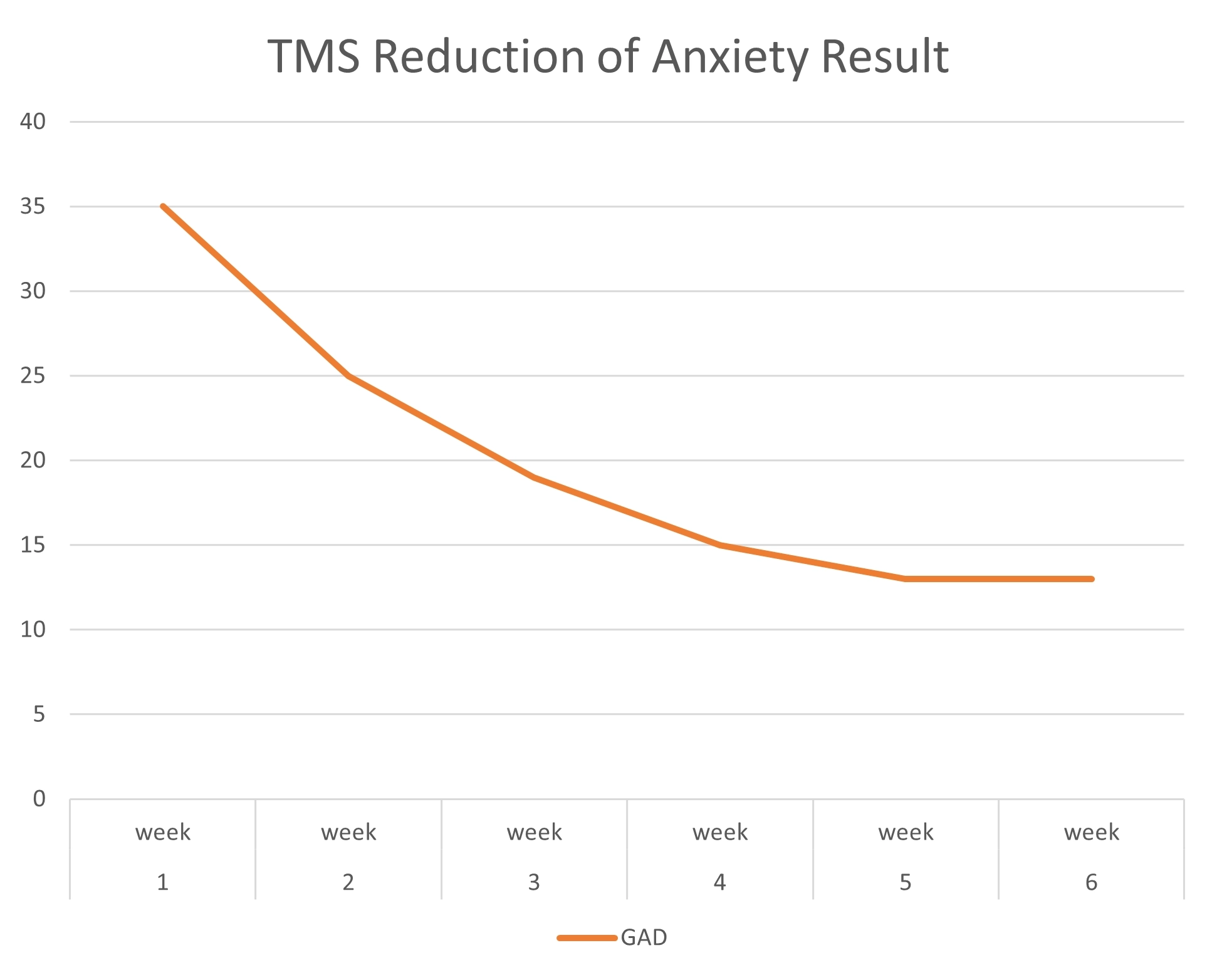
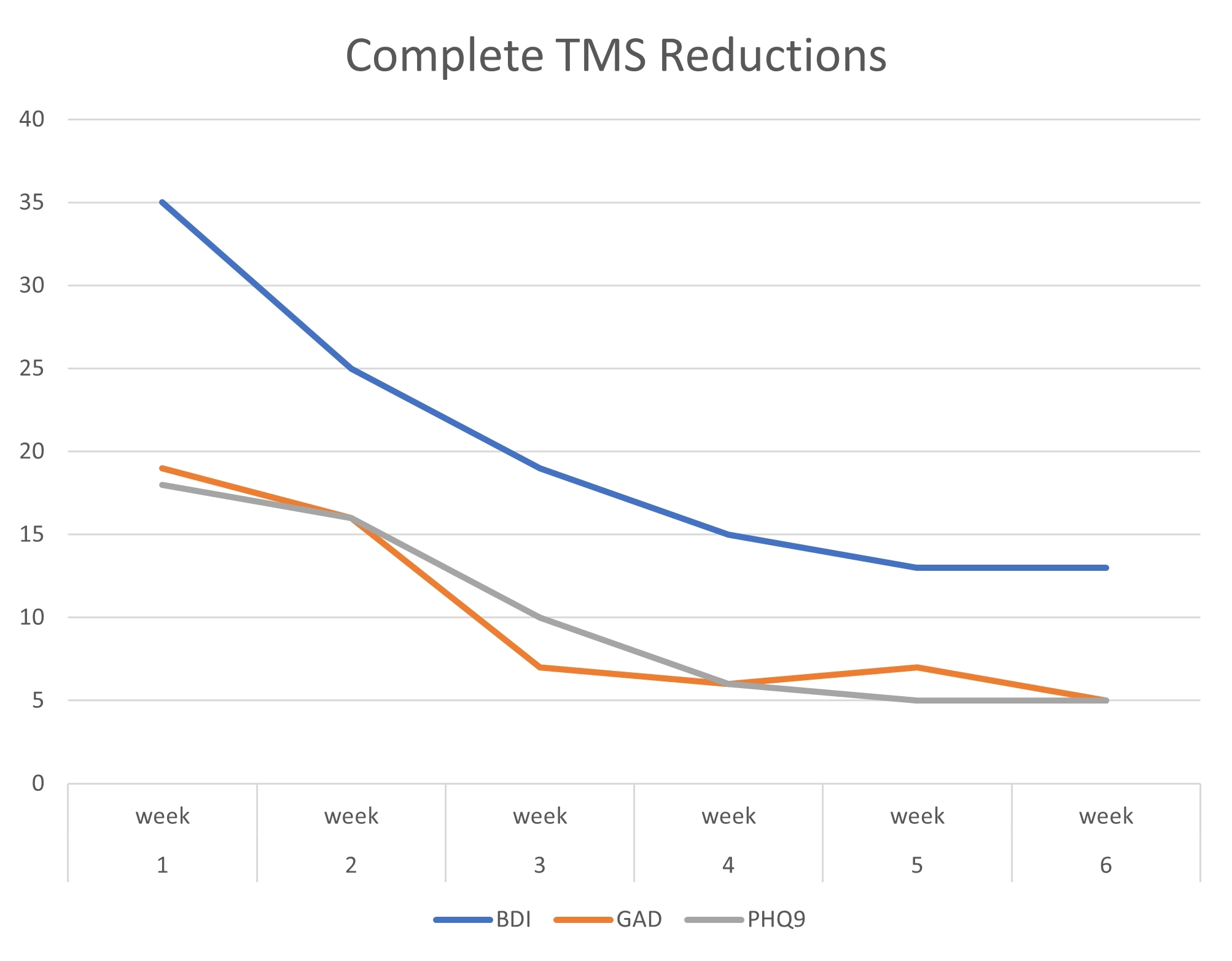
TMS Studies & Clinical Results
Get an inside look at DiscoveryMD’s Dr. Maria Davila’s TMS studies and trainings, as well as clinical results from outside TMS studies.
Patient Stories & Media
Brain Wellness Institute in Costa Mesa Partners with DAN MED TMS to help patients transform their lives with TMS
Get Help Today
Get in touch with DiscoveryMD via phone or filling out the form below.
- Kessler RC, et al. (2003). JAMA.
- https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml, accessed 1/16/2018.
- Gaynes BN et al. (2008). Cleveland Clinic Journal of Medicine. 75(1): 57-66.